Maximizing the Effectiveness of Mounjaro Injections: Best Practices
As nurses, mastering new treatments like MOUNJARO, an innovative medication for type 2 diabetes mellitus, is essential. This concise guide will delve into MOUNJARO’s workings, its usage, contraindications, and potential side effects.
It’s all about empowering you, the nurse, to administer this novel drug safely and effectively. Let’s explore how MOUNJARO is shaping diabetes care.
What is Mounjaro?
Mounjaro is a new medication specifically designed for adults with type 2 diabetes mellitus. As a glucose-dependent insulinotropic polypeptide (GIP) receptor and glucagon-like peptide-1 (GLP-1) receptor agonist, Mounjaro works to lower fasting and postprandial glucose concentration, reduce food intake, and bind to and activate both the GIP and GLP-1 receptors.
This medication is injected subcutaneously once weekly, with a recommended starting dosage of 2.5 mg. Its active ingredient, tirzepatide, enhances first- and second-phase insulin secretion, reduces glucagon levels in a glucose-dependent manner, and lowers glucose concentrations.
Understanding the Mechanism of Action of Tirzepatide in MOUNJARO
Tirzepatide, the active ingredient in MOUNJARO, selectively binds to and activates both the GIP and GLP-1 receptors. Understanding this interaction can shed light on MOUNJARO’s mechanism of action.
Tirzepatide enhances insulin secretion, reduces glucagon levels in a glucose-dependent manner, and lowers fasting and postprandial glucose concentrations. It also impacts food intake, which could lead to weight loss in patients with type 2 diabetes mellitus.
The Benefits of Tirzepatide in Glycemic Control and Weight Reduction
The effectiveness of Tirzepatide extends to improving glycemic control, reducing weight, and lowering the risk of cardiovascular disease. Clinical trials have demonstrated its effectiveness in reducing HbA1c levels, making it more beneficial than a placebo and other antidiabetic medications.
Tirzepatide’s ability to lower the risk of hypoglycemia compared to other antidiabetic medications makes it a favorable option for patients with type 2 diabetes mellitus. The multitude of benefits from this single drug highlights its potential in managing type 2 diabetes effectively.
Preparing to Inject Mounjaro

Before administering Mounjaro, there are several critical steps to take to ensure both the safety and appropriateness of the medication for the patient.
- Patient Medical History: Review the patient’s medical history to rule out any contraindications for Mounjaro. If the patient has a personal or family history of medullary thyroid carcinoma (MTC) or Multiple Endocrine Neoplasia syndrome type 2 (MEN 2), Mounjaro should not be administered. Similarly, patients with a history of pancreatitis, severe gastrointestinal disease, or type 1 diabetes mellitus are not suitable candidates for Mounjaro.
- Review for Hypoglycemia and Hypersensitivity: Review the patient’s medical records for any history of severe hypoglycemia or hypersensitivity reactions. Mounjaro has been associated with hypoglycemia and hypersensitivity reactions in some patients, so be sure to discuss these potential risks and any previous occurrences with the patient.
- Medication Review: Evaluate the patient’s current medications. Mounjaro can interact with other drugs, especially insulin secretagogues or insulin, potentially increasing the risk of hypoglycemia. If the patient is on these medications, discuss the risk of hypoglycemia and ensure that blood glucose levels are closely monitored.
- Gather the Necessary Supplies: Before beginning the injection, gather all the necessary supplies. This includes:
- Mounjaro medication
- Sterile needle and syringe (for drawing and administering the medication)
- Alcohol wipes (for cleaning the injection site)
- A sharps container (for safely discarding the used needle and syringe)
- Sterile bandages or cotton balls (in case of bleeding post-injection)
By following these steps, nurses can effectively prepare for the safe administration of Mounjaro to eligible patients with type 2 diabetes.
Choosing an Injection Site
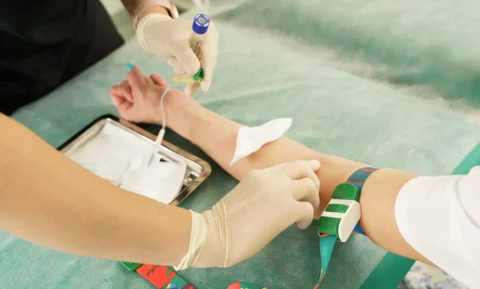
Choosing an appropriate injection site is critical for the subcutaneous administration of Mounjaro. It can be injected in the abdomen, thighs, or the back of the upper arms. Ensure to rotate injection sites to minimize skin irritation and avoid administering the injection at the exact same spot each time. Avoid areas with wounds, scars, or skin irritation.
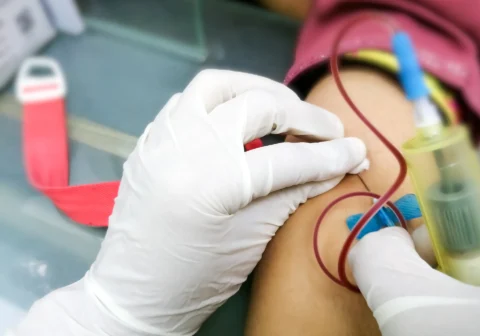
Step-by-Step Guide to Inject Mounjaro
Proper administration of Mounjaro is critical to ensure its efficacy and safety. Here’s a detailed guide to injecting Mounjaro, including dosage instructions.
Step 1
Wash your hands thoroughly and gather the Mounjaro medication, alcohol wipes, and a sterile needle and syringe. Visually inspect the Mounjaro solution for any particles or discoloration; if observed, do not use.
Step 2
Choose an injection site and clean it with an alcohol wipe. Allow it to air dry.
Step 3
Fill the syringe with the appropriate dosage of Mounjaro. The recommended starting dosage is 2.5 mg injected subcutaneously once weekly, with a maximum of 15 mg per week. Always adhere to the specific dosage prescribed by the healthcare provider.
Step 4
Pinch the skin gently and insert the needle at a 45 to 90-degree angle, ensuring the medication is administered subcutaneously. Inject the medication slowly and completely.
Step 5
Withdraw the needle gently and promptly. If necessary, use a sterile bandage or cotton ball to manage any minor bleeding at the injection site.
Understanding the Contraindications of MOUNJARO
Patients intending to use MOUNJARO need to be aware of certain contraindications that may affect their health. A key contraindication is a personal or family history of medullary thyroid carcinoma (MTC) or Multiple Endocrine Neoplasia syndrome type 2 (MEN 2).
This is because studies have suggested a link between MOUNJARO and the development of thyroid C-cell tumors in rats. While it remains uncertain whether MOUNJARO can cause thyroid C-cell tumors in humans, this risk factor necessitates caution.
Another important factor to consider is the role of routine monitoring of serum calcitonin or the use of thyroid ultrasound. These tests are typically used for early detection of MTC in patients using drugs like MOUNJARO.
Pancreatitis Risk
Individuals with a history of pancreatitis should avoid using MOUNJARO. The medication isn’t recommended for these patients due to the potential health risks. Furthermore, if a patient using MOUNJARO develops symptoms that suggest the onset of pancreatitis, the drug should be discontinued immediately.
Type 1 Diabetes and Gastrointestinal Conditions
MOUNJARO isn’t suitable for patients with type 1 diabetes mellitus or severe gastrointestinal disease. Its impact hasn’t been sufficiently studied in these groups, so it’s safer to avoid its use.
Similarly, MOUNJARO has not been tested on patients with non-proliferative diabetic retinopathy requiring acute therapy, proliferative diabetic retinopathy, or diabetic macular edema, making its effects on these conditions uncertain.
Cholelithiasis
For patients with cholelithiasis, or gallstones, it’s essential to have gallbladder studies and regular clinical follow-ups. This is due to the incidence of acute gallbladder disease observed in clinical trials involving MOUNJARO.
Adverse Reactions to MOUNJARO
It’s crucial to understand that while MOUNJARO can be an effective treatment for type 2 diabetes, it may also lead to some adverse reactions.
Common issues reported by patients include nausea, diarrhea, decreased appetite, vomiting, constipation, dyspepsia, and abdominal pain. These gastrointestinal side effects occurred more frequently in patients receiving MOUNJARO than a placebo.
Additionally, hypoglycemia, a condition characterized by low blood sugar levels, was observed more frequently when MOUNJARO was used in combination with sulfonylurea. This suggests careful monitoring of blood glucose levels is necessary when using this combination of drugs.
In some cases, patients treated with MOUNJARO experienced an increased heart rate, with an average rise of 2 to 4 beats per minute. A small number of MOUNJARO-treated patients also reported reactions at the injection site, while a small percentage reported the onset of acute gallbladder disease.
Addressing Common Concerns About MOUNJARO Usage
When contemplating a treatment regimen that includes MOUNJARO, a variety of questions may arise. Let’s clarify some of the most common concerns.
The Risks with MOUNJARO and Other Antidiabetic Medications
A crucial point to be aware of is the increased risk of hypoglycemia when MOUNJARO is used in combination with insulin or insulin secretagogues. This combination requires vigilant monitoring of blood sugar levels and immediate discontinuation if severe hypoglycemia is suspected.
Potential Impact of MOUNJARO on Other Medications
Another important consideration when using MOUNJARO is its potential impact on the absorption of other medications taken orally. Due to MOUNJARO’s ability to delay gastric emptying, it may influence the effectiveness of other medications.
This is particularly relevant for patients with hepatic impairment, where drug interactions could alter MOUNJARO metabolism and increase the risk of adverse effects.
Using MOUNJARO During Pregnancy and Breastfeeding
For women who are pregnant or breastfeeding, the decision to use MOUNJARO should be made after a thorough consultation with a healthcare provider.
The safety and effectiveness of MOUNJARO have not been established for these populations. Additionally, MOUNJARO may affect the efficacy of oral hormonal contraceptives due to its action on gastric emptying.
Implementing Semaglutide and Tirzepatide into Your Aesthetic Practice
Embrace the future of weight management in your aesthetic practice by integrating the cutting-edge medications Semaglutide and Tirzepatide.
We are delighted to present our exclusive online course: “Implementing Semaglutide and Tirzepatide into Your Aesthetic Practice“
This program is your key to unlocking untapped opportunities within your practice!
This course is spearheaded by Nikki Plourde, NP-C, a seasoned healthcare professional with over ten years of experience in Family Medicine and a proven track record in deploying successful weight management treatments. With her guidance, you’ll navigate the complexities of establishing an effective weight management program.
By joining this program, you’ll gain:
- An in-depth knowledge of Semaglutide and Tirzepatide, their uses, contraindications, operating mechanisms, potential side effects, dosages, and monitoring protocols.
- Access to a detailed online curriculum featuring a recorded PowerPoint presentation brimming with vital information.
- Guidelines on converting dosages between Semaglutide and Tirzepatide.
- A hands-on tutorial on medication ordering, supported by a practical online example.
- Creative business and marketing strategies complemented by a cost analysis and return-on-investment evaluation.
- Detailed advice on patient consultations and follow-ups, inclusive of pre-appointment questionnaire suggestions.
- A resource kit filled with example protocols, consent forms, SOAP notes, patient questionnaires/handouts, and medication safety documentation.
Furthermore, as a bonus, course enrollees are entitled to group rate pricing for Semaglutide!
Don’t allow your practice to fall behind. Equip yourself with the requisite skills and resources to provide an all-encompassing weight management service and watch your practice flourish. With continual support, your success is within reach.
Grab this chance now! Take a proactive step towards transforming your aesthetic practice today!