Tirzepatide and Ozempic Compared: An In-Depth Explanation
So how exactly does Tirzepatide go about lowering blood sugar and reducing appetite? Let’s break down step-by-step how this diabetes medication works its magic.
First, some background:
Tirzepatide is what’s known as a dual GIP and GLP-1 receptor agonist. In plain English, this means it activates two types of receptors in the body: GIP receptors and GLP-1 receptors.
GIP stands for glucose-dependent insulinotropic polypeptide. When GIP binds to its receptors, it stimulates the pancreas to release insulin after a meal. GLP-1 stands for glucagon-like peptide-1.
When GLP-1 binds its receptors, it suppresses the release of glucagon (a hormone that raises blood sugar) and slows the emptying of the stomach.
So by binding to and activating both GIP and GLP-1 receptors, Tirzepatide sets off a chain of events in the body. It causes the pancreas to secrete more insulin in a glucose-dependent manner – meaning only when blood sugar levels are high after eating.
It suppresses the release of glucagon from the pancreas. And finally, it slows down how fast food empties from the stomach.
The end result? Lowered blood sugar levels after meals and throughout the day. The slowed gastric emptying also makes you feel fuller longer, which can help reduce caloric intake and promote weight loss.
Monitoring Tirzepatide Treatment
For Tirzepatide to work its magic against high blood sugar levels and excess weight, proper monitoring of therapy is key.
Healthcare providers will track several things during a patient’s course of Tirzepatide treatment:
Hemoglobin A1c levels
As discussed previously, Tirzepatide has been shown to significantly lower hemoglobin A1c levels, an indicator of average blood glucose over the past 2-3 months.
Providers will check hemoglobin A1c levels before starting Tirzepatide and then every 3 months or so to gauge how well the medication is working and if any dose adjustments are needed. A hemoglobin A1c drop of 1% or more within 3-6 months is generally considered a good response.
Body weight
Weight loss is a common side effect of Tirzepatide treatment due to its impact on appetite and gastric emptying.
Healthcare providers will monitor patients’ weight at follow-up visits to evaluate the effectiveness of the medication for weight management and detect any concerning weight loss. An average loss of 5-10% of body weight over a few months is generally expected.
Gastrointestinal symptoms
Since nausea, vomiting, diarrhea, and constipation are the most frequently reported side effects of Tirzepatide, providers will ask patients about the presence and severity of these symptoms, especially as doses increase. Early detection and management of side effects are important for treatment adherence.
Blood tests
Baseline and periodic blood tests are recommended to check for asymptomatic changes, such as elevated pancreatic enzymes or abnormalities in kidney or thyroid function that require adjusting Tirzepatide therapy. However, routine monitoring of calcitonin levels is not advised.
Dosage Guidelines for Initiating Tirzepatide Therapy
Proper dosing of Tirzepatide is important to achieve optimal glycemic control and weight loss while minimizing side effects. Here are the general dosage guidelines for initiating and titrating Tirzepatide therapy:
Start with an initial dose of 5 mg, administered as a subcutaneous injection once weekly. The 5 mg dose is generally well tolerated with a low risk of side effects.
After at least 4 weeks at the initial 5 mg dose, patients can increase to the next dose level of 7.5 mg if target blood sugar and weight goals have not been met and there are no tolerability issues.
Dose increases can then be made in increments of 2.5 mg every 4 weeks, up to the maximum 15 mg weekly dose.Higher doses have been associated with greater reductions in hemoglobin A1c and body weight but also a higher risk of side effects.
The maximum recommended 15 mg weekly dose should only be used in patients who tolerated the lower doses well and require additional glycemic and weight loss benefits.
Patients should be carefully monitored for side effects, especially gastrointestinal symptoms, as doses increase. Some patients may require a reduction to the previous well-tolerated dose.
After the maximum tolerated dose is achieved, it is generally continued long-term to maintain treatment benefits. Any future dose adjustments are based on efficacy and tolerability.
Adjusting the Dose of Tirzepatide
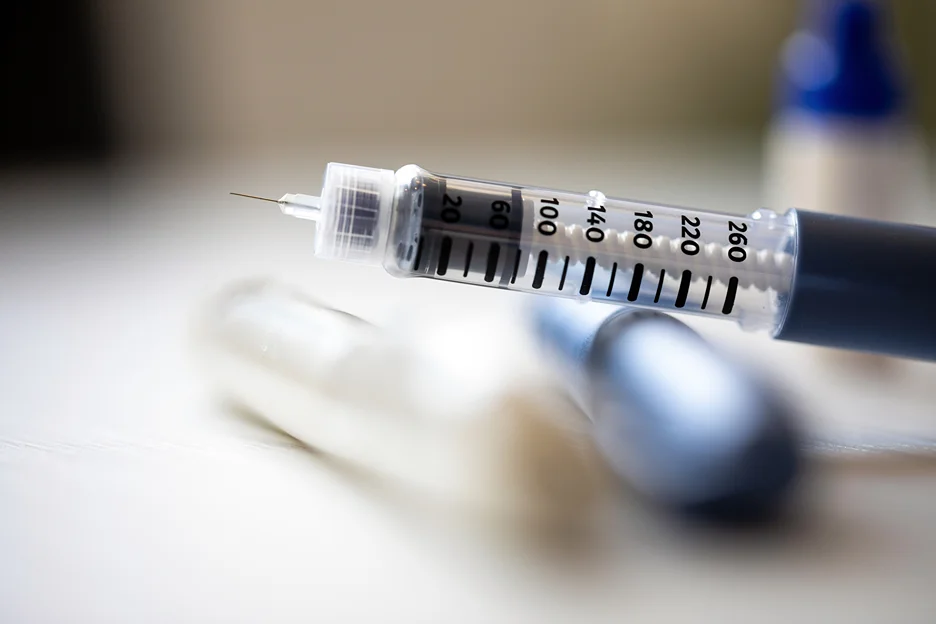
The initial dose of tirzepatide is typically 5 mg once weekly. However, doses can be increased based on how well the patient responds to therapy and what side effects they experience.
The standard dosing schedule for tirzepatide involves increasing the dose by 2.5 mg increments after at least 4 weeks at the current dose. Here is what the titration schedule typically looks like:
- Week 1 to 4: 5 mg once weekly
- Week 5 to 8: Increase to 7.5 mg once weekly if glucose and weight goals are not met and side effects are tolerable
- Week 9 to 12: Increase to 10 mg once weekly if further glucose and weight reductions are needed
- Week 13 to 16: Increase to 12.5 mg once weekly, and so on
- Maximum dose: 15 mg once weekly
The key factors healthcare providers consider when adjusting a patient’s tirzepatide dose include:
Hemoglobin A1c: If A1c levels have not decreased by at least 1% after 3 months, increasing the dose may help achieve better glucose control.
Body weight: For patients who desire more weight loss, escalating the dose can produce additional weight reduction.
Side effects: Patients who experience only mild to moderate gastrointestinal symptoms at a given dose may tolerate an increase. However, dose reductions may be needed for severe side effects.
Treatment goals: Providers consider the individualized treatment goals of each patient, whether that be stricter glucose control, more weight loss, fewer side effects, or a combination.
Safety Considerations and Monitoring for Tirzepatide
While tirzepatide has been shown to effectively improve glycemic control and promote weight loss in patients with type 2 diabetes, it is important to remain aware of safety considerations and monitor patients closely during treatment.
Common side effects such as nausea, diarrhea and vomiting can potentially lead to dehydration, so patients should be monitored for signs and symptoms of fluid loss.
Healthcare providers should educate patients on rehydration strategies and when to seek immediate medical attention.
There have been rare reports of acute kidney injury associated with tirzepatide use. As a result, baseline and periodic monitoring of kidney function through blood tests is recommended, especially in patients with pre-existing chronic kidney disease.
Tirzepatide dosage may need to be adjusted or discontinued in cases of significant deterioration in kidney function.
Patients should be advised to report any hypersensitivity reactions, particularly at the injection site. These types of reactions have been infrequently associated with tirzepatide. Patients with a history of hypersensitivity to other GLP-1 receptor agonists may be at higher risk and should be monitored closely.
While routine monitoring of calcitonin levels is not required, patients should be advised of the potential risk of medullary thyroid cancer associated with tirzepatide and similar drugs.
Healthcare providers should remain alert for any suspicious symptoms and consider appropriate follow-up testing if medullary cancer is suspected.
Certain safety risks have been reported with tirzepatide though they are relatively rare. Close patient monitoring during treatment, especially for side effects, kidney function, hypersensitivity reactions and potential medical risks, can help ensure the safe and effective use of this novel diabetes medication.
Healthcare providers play an important role in educating patients about how to maximize benefit while minimizing harm with tirzepatide therapy.
Contraindications and Warnings for Tirzepatide
There are certain medical conditions and other factors which make tirzepatide inappropriate or unsafe for some patients. Healthcare providers must carefully review a patient’s medical history and current health status to determine whether tirzepatide is contraindicated.
Contraindications for tirzepatide include:
Personal or family history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2 (MEN 2).
Tirzepatide and other drugs in its class have been associated with the development of calcitonin-positive medullary thyroid cancer in rats and mice. Therefore tirzepatide is contraindicated in patients at higher risk for this rare thyroid cancer.
Other thyroid cancer-related risk factors. Patients with risk factors such as radiation exposure to the head and neck, lymphoma of the head and neck, or preexisting benign thyroid nodules are at increased risk for thyroid cancer and should not use tirzepatide.
Hypersensitivity to tirzepatide or any of its ingredients. Patients who have had an allergic reaction to tirzepatide in the past should not receive this medication as significant hypersensitivity reactions may reoccur.
Warnings and precautions for tirzepatide include:
Pancreatitis. Patients with a history of pancreatitis, pancreatic adenoma, or elevated pancreatic enzyme levels should be closely monitored to prevent exacerbation of these conditions. Use in patients with a prior history of pancreatitis is not recommended.
Hypoglycemia. Combination use with insulin or insulin secretagogues may increase the risk of hypoglycemia. Dosage adjustments and increased glucose monitoring may be needed.
There are certain medical conditions, risk factors and sensitivities which necessitate avoiding tirzepatide or utilizing it with caution under close medical supervision.
Healthcare providers should thoroughly screen patients for contraindications and regularly monitor for warning signs during therapy.
FAQs: Tirzepatide vs Ozempic
Who is tirzepatide for?
Adults with type 2 diabetes who need better control of their blood sugar and weight loss. Tirzepatide targets two receptors in the body involved in controlling blood glucose and food intake.
How does tirzepatide work to treat type 2 diabetes?
Tirzepatide is a glucagon-like peptide-1 (GLP-1) receptor agonist. It works like natural GLP-1 hormones to stimulate insulin release, slow gastric emptying, and reduce glucagon and food intake. These effects help lower blood sugar and promote weight loss.
What is the active ingredient in tirzepatide?
The active ingredient in tirzepatide is a dual agonist of the glucose-dependent insulinotropic polypeptide receptor and GLP-1 receptor. It has a similar mechanism of action as other GLP-1 medications.
How often do you inject tirzepatide?
Currently, tirzepatide is only available as a once-weekly injection that patients self-administer subcutaneously. The frequency of injections may change if higher or lower doses are needed.
Can tirzepatide cause side effects on blood pressure or heart health?
In clinical trials, there were no significant changes in blood pressure or issues related to heart health observed with tirzepatide. However, some people may experience side effects like abdominal pain, nausea, vomiting, and diarrhea that could theoretically impact blood pressure. Close monitoring is important.